Waste heat, the byproduct of all kinds of machinery and processes, has long been seen as a potential source of energy – if only the capture-and-convert process could be made more efficient.
At Northwestern University, researchers think they’ve pulled it off.
Their apparent heat-recovery breakthrough is written up in the journal Nature Chemistry in a paper titled, “Strained endotaxial nanostructures with
high thermoelectric figure of merit.”
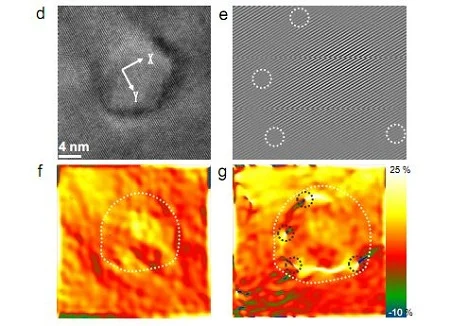
Thankfully, the university has summed up the findings in a more digestible form, explaining that nanocrystals of rock salt placed into lead telluride was the key.
It seems that earlier attempts at this kind of “nanoscale inclusion in bulk material” had boosted the energy conversion efficiency of lead telluride, but because they also scattered electrons, had the unhappy side-effect of decreasing overall conductivity.
“In this study,” the university reports, “the Northwestern team offers the first example of using nanostructures in lead telluride to reduce electron scattering and increase the energy conversion efficiency of the material.”
What’s it mean in terms of real-world application?
Vinayak Dravid, a co-author of the paper, provided a simple way to understand that: ”We can put this material inside of an inexpensive device with a few electrical wires and attach it to something like a light bulb. The device can make the light bulb more efficient by taking the heat it generates and converting part of the heat, 10 to 15 percent, into a more useful energy like electricity.”
So any industry that uses heat to make products – cars, chemicals, bricks, glass – could take advantage of this technology to make their systems more efficient, the researchers said.






