A couple of grains of salt could be all that’s needed to help bacteria produce hydrogen from wastewater or organic byproducts.
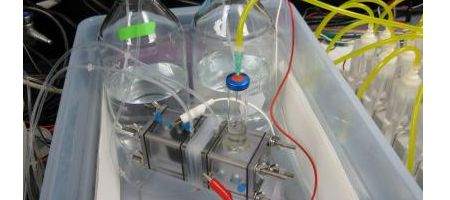
“This system could produce hydrogen anyplace that there is waste water near sea water,” says Bruce Logan of Penn State University.
“It uses no grid electricity and is completely carbon neutral. It is an inexhaustible source of energy.”
In the past, microbial electrolysis cells have required some electrical input. Now, though, the Penn team’s discovered that the extra energy can be delivered simply through the difference between river water and seawater.
Pure hydrogen gas is the result.
The cells have an efficiency of between 58 and 64 percent, and produced between 0.8 to 1.6 cubic meters of hydrogen for every cubic meter of liquid passing through the cell each day.
The researchers estimate that only about one percent of the energy produced in the cell is needed to pump water through the system.
The key is reverse-electrodialysis, RED, a process that extracts energy from the ionic differences between salt water and fresh water. A RED stack consists of alternating ion exchange membranes – positive and negative – with each RED contributing to the electrical output.
For RED technology to split water requires 1.8 volts. This would normally require about 25 pairs of membranes and increase pumping resistance.
However, combining RED technology with bacteria that consume organic material and produce an electric current, means only five membrane pairs are needed to produce hydrogen.
“The added voltage that we need is a lot less than the 1.8 volts necessary to hydrolyze water,” says Logan.
“Biodegradable liquids and cellulose waste are abundant, and with no energy in and hydrogen out we can get rid of wastewater and by-products. This could be an inexhaustible source of energy.”






