The Martian atmosphere contains water vapor in a supersaturated state, say surprised ESA scientists.
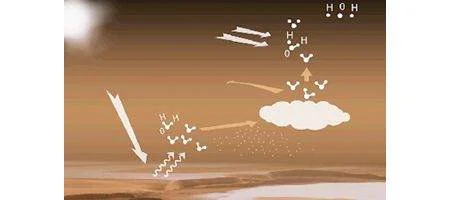
On Earth, water vapor tends to condense into a liquid when the temperature falls below dewpoint. At this point, the atmosphere is said to be ‘saturated’, since it can’t hold any more moisture at that temperature and pressure.
However, condensation can sometimes be much slower, especially when particles around which the water molecules can coalesce are scarce. The excess water vapor then remains in the gaseous state, in a phenomenon known as supersaturation.
Until now, it was assumed that supersaturation couldn’t occur in the Martian atmosphere – but ESA says that data from the Mars Express spacecraft leaves no room for doubt.
Surprisingly, while several spacecraft have visited Mars, most of their instruments have been focused on surface data. The way in which water content on Mars varies with height remained largely unexplored.
But observations by the Mars Express’ SPICAM spectrometer on board the Mars Express spacecraft has now done just that, observing light from the sun as it travels through the Martian atmosphere at sunrise and sunset.
Contrary to previous belief, the researchers discovered that water vapor supersaturation is a frequent phenomenon on Mars. Indeed, they found levels of supersaturation that were up to ten times greater than on Earth.
“This ability of water vapor to exist in a highly supersaturated state would, for example, [be able] to supply the southern hemisphere of Mars with water, far more efficiently than models currently predict,” says Franck Montmessin, SPICAM project leader.
Moreover, a far greater quantity of water vapor than thought may be transported high enough in the atmosphere to be destroyed by photodissociation.
If this really is the case, there would be consequences for the whole study of Martian water, much of which is known to have continually escaped to space for billions of years.






