It sometimes seems as if there isn’t anything that can’t be done better with graphene. Now, researchers at Rensselaer Polytechnic Institute say that the stuff can outperform leading commercial gas sensors in detecting potentially dangerous and explosive chemicals.
Their new sensor has successfully and repeatedly measured ammonia (NH3) and nitrogen dioxide (NO2) at concentrations as small as 20 parts-per-million.
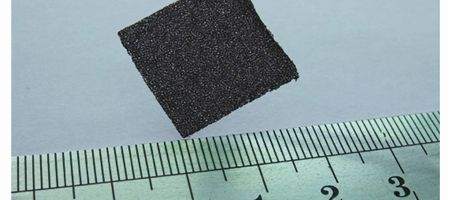
Made from continuous graphene nanosheets that grow into a foam-like structure about the size of a postage stamp and thickness of felt, the sensor is flexible and rugged.
“We are very excited about this new discovery, which we think could lead to new commercial gas sensors,” says Rensselaer engineering professor Nikhil Koratkar.
“So far, the sensors have shown to be significantly more sensitive at detecting ammonia and nitrogen dioxide at room temperature than the commercial gas detectors on the market today.”
The team grew graphene on a structure of nickel foam, which was then removed to leave a large, free-standing network of foam-like graphene. The walls of the sensor are comprised of continuous graphene sheets without any physical breaks or interfaces between the sheets.
When the graphene foam is exposedto air contaminated with trace amounts of ammonia or nitrogen dioxide, the researchers found that the gas particles stuck, or adsorbed, to the foam’s surface. This change in surface chemistry has a distinct impact upon the electrical resistance of the graphene, which can be measured to detect different gases.
It can detect ammonia or nitrogen dioxide at 1,000 parts-per-million in under 10 minutes at room temperature and atmospheric pressure – ten times more sensitive than traditional sensors under the same conditions. Indeed, it’s effective down to 20 parts-per-million, much lower than commercially available devices.
And while many commercially available devices require high power consumption, the graphene foam detector operates at room temperature.
“We see this as the first practical nanostructure-based gas detector that’s viable for commercialization,” says Koratkar.
“Our results show the graphene foam is able to detect ammonia and nitrogen dioxide at a concentration that is an order of magnitude lower than commercial gas detectors on the market today.”
The graphene foam can be engineered to detect many different gases beyond ammonia and nitrogen dioxide, he says.






