AstraZenica’s Covid-19 vaccine could be made available by December this year. This was announced by the British pharmaceutical’s CEO, Pascal Soriot. Currently, the vaccine is on it’s third phase of clinical trials in the U.K, U.S and some other countries. Once the results of the last stage of trials prove that the vaccine is safe and effective against coronavirus, regulators can endorse it for large-scale use. Last month, the vaccine was shown to produce an immune response on older adults.
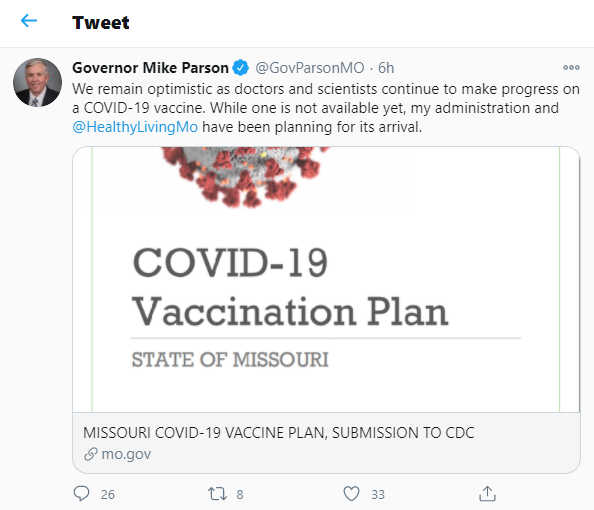
The Hill: “We would hope that large-scale vaccinations would be possible starting in January next year — possibly even December,” says CEO Pascal Soriot

According to The Hill, Results from the late-stage trials that include tens of thousands of participants are expected to be released in the coming weeks. Once the data is reported, regulators can approve it for widespread use if it’s shown to be safe and effective.
AstraZeneca Chief Executive Officer Pascal Soriot said the U.S. Food and Drug Administration (FDA) may want to wait for the results from the U.S. trial, which was paused in September as other trials around the world continued, before reviewing the vaccine and giving it approval. AstraZeneca said it can provide hundreds of millions of doses on a “rolling basis” once the shot is approved.