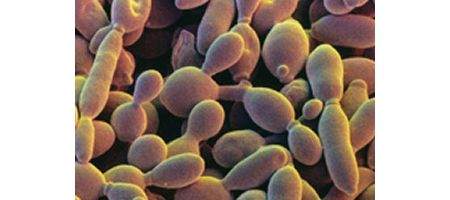
A team of Johns Hopkins researchers has created from scratch a new form of yeast that includes synthetic DNA.
It allows scientists to rearrange the yeast’s genetic material at will.
“We have created a research tool that not only lets us learn more about yeast biology and genome biology, but also holds out the possibility of someday designing genomes for specific purposes, like making new vaccines or medications,” says Jef D Boeke of the University School of Medicine.
Using the already-known full genetic code of the yeast genome as a starting point, graduate student Sarah Richardson wrote a software program for making a series of systematic changes to the DNA sequence.
The changes were intended to remove some of the repetitive and less-used regions of DNA between genes, and to generate a mutated ‘version 2.0’ of a yeast cell’s original 9R chromosome.
The smallest chromosome arm in the yeast genome, 9R contains about 100,000 base pairs of DNA and represents about one percent of the single-celled organism’s genome.
To build the actual chromosome started with stringing individual bases of DNA together and then assembling them into longer segments. Finally,segments of about 10,000 base pairs were put into live yeast cells and essentially swapped for the existing counterpart in the chromosome.
As well as 9R, the team also made a smaller piece of the chromosome 6L.
When yeast cells containing the synthetic chromosomes were tested for their ability to grow on different nutrients and in different conditions, they came out indistinguishable from natural yeast every time.
Key to the achievement is an ‘inducible evolution system’ called SCRaMbLE – Synthetic Chromosome Rearrangement and Modification by Lox-P mediated Evolution.
“We developed SCRaMbLE to enable us to pull a mutation trigger — essentially causing the synthetic chromosome to rearrange itself and introducing changes similar to what might happen during evolution, but without the long wait,” says Boeke.
It allows a number of variables to be changed simultaneously.
The team activated SCRaMbLE in yeast containing both the synthetic 9R and 6L chromosomes, then analyzed the DNA from the yeast cells.
When fed various nutrients, they grew at different rates, and some of the fast-growing organisms had very specific defects resulting from specific gene loss – showing that SCRaMbLE does indeed introduce random variation.
When the team analyzed the molecular structure of the synthetic 9R and 6L chromosomes from this SCRaMbLEd population, they found chromosomes with small deletions, rearrangements, and other alterations, at wildly varying locations.
“If you think of the yeast genome as a deck of cards, we now have a system by which we can shuffle it and/or remove different combinations of 5,000 of those cards to get lots of different decks from the same starter deck,” Boeke says.
“By shuffling the DNA according to our specifications, we hope to be able to custom design organisms that perhaps will grow better in adverse environments, or maybe make one percent more ethanol than native yeast.”
Boeke says the ultimate goal of the project is to synthesize the whole yeast genome – about 6,000 genes – and SCRaMbLE the 5,000 likely to be individually dispensable.
He says he’s making the tool available freely, without intellectual property protection.






